Sony does the VTC series.
3 Likes
This is so true. Graphs can tell 1 thing but real life experience may be something very different. That’s why these forums are a great way to exchange experience.
By the way, I actually own a couple of those 3500mah Fogstar batteries (the first ones I ever bought, I knew nothing) and they are pretty good when used correctly. I bought them for an Aspire MTL device and I’ve used them in a 3-battery mod using RDA’s/RTA’s vaping around 60-65W (so well within its limits). They last about as long between charges as the Sony VTC6’s (3000mah, 20A) or even the Samsung 30Q’s (3000mah, 20A) but the latter are MUCH safer.
When high capacity batteries are drained at max rate, they don’t last any longer than lower capacity batteries that are drained well below max rate. That is real life experience that I’ve never seen in any graph.
3 Likes
Thanks @Kinnikinnick, dropping bigger pics…
4 Likes
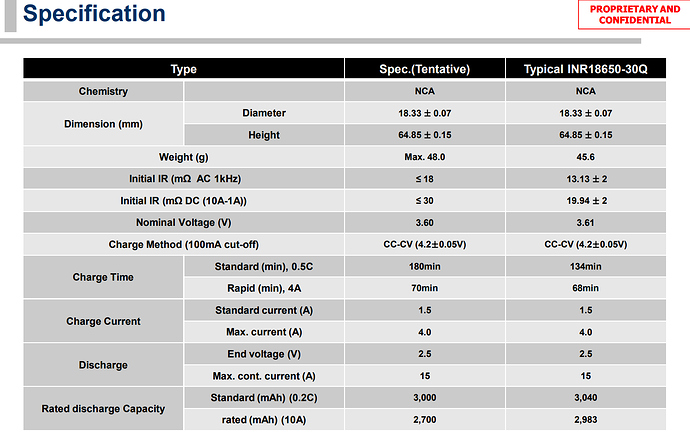
BTW, the Samsung 30Q is (from what I can see) rated at a 15 Ampere continuous discharge current:
https://lygte-info.dk/review/batteries2012/Samsung%20INR18650-30Q%203000mAh%20(Pink)%20UK.html
I was reading a seemingly fairly impressive treatise regarding Lithium-Ion batteries, and it was stated that the higher the intended battery current capacity (in total mAH), the less “stable” is the design. This would follow - in that the (inner) wraps are constructed of thinner material in the higher capacity efforts, allowing for a higher probability of unintended “dendritic” electrical-short connections to form internally.
Having purchased no less than 10 of these little LG MJ1 pigs (at $3 each from the pompous jerks at IMR Batteries in a “closeout” sale), I am hoping that my limited to <2.5 Ampere continuous battery discharge currents, and conservative (500 mAH) charging regimens will allow me to get some useful duty out of these batteries without “safety incidents”. Experience any “incidents” with your “Fogstars” ?
1 Like
I go by Mooch’s recommendation:

Usually, my batteries operate below 75C (and I stay mostly below or around 15A anyway).
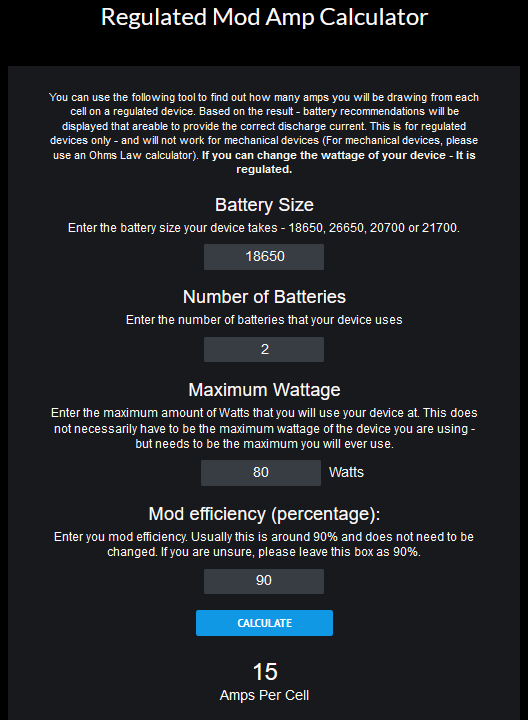
As long as you have a 2 battery regulated mod, don’t go over 80W, or a 3 battery mod even better, you shouldn’t worry about the 30Q’s in any case.
2 Likes
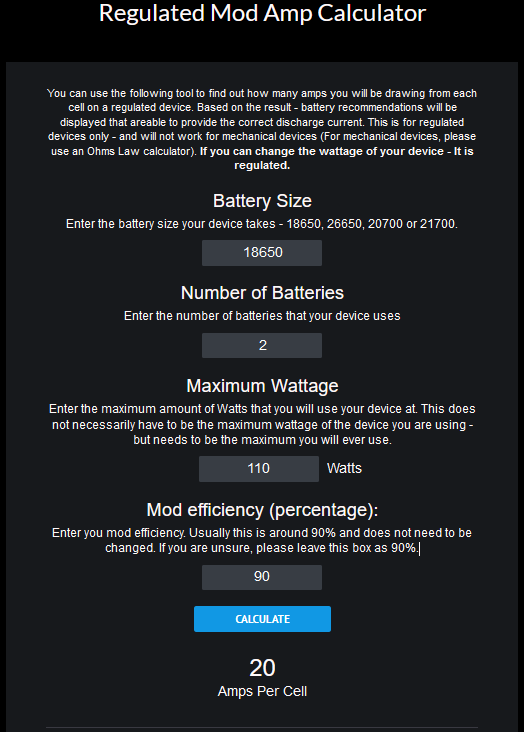
Right. But the reason that I mentioned this is that you posted a 20 Ampere current rating for the 30Q:

2 Likes
Because that is Mooch’s test result, not the manufacturer’s specification (which uses the 75C clause in mind probably).
Up to 110W on 2 battery regulated devices, the Samsung 30Q’s will operate very happily. But that is not to say there aren’t any better quality batteries for this purpose. Just saying they’re a relative safe option and definitely good quality/price ratio for people pinching the pennies.
If you want my recommendation purely based on quality and safety, I will only say go for the Molicels P26As, if only that they’re the
only manufacturer that says their batteries are OK for vaping.
2 Likes
The (only) Samsung generated specifications document that I have located states 15 Amperes Max:
Source: https://eu.nkon.nl/sk/k/30q.pdf
1 Like
Uhuh, but Samsung also recommends NOT using its batteries for vaping 
So if you just go by their recommendation, why even think about them

2 Likes
Suit yourself there. I would myself strangely choose to consider Samsung’s published specifications.

Despite that lovely sentiment (as was enunciated by “The Mooch”, with the best of intentions), I myself was unable to locate any US-based retailer of Molicels batteries who would honor that. Do you know of (any) specific retailers (located anywhere in the world) selling Molicel batteries to consumers who do replace (such openly used by the consumer for “vaping” rituals) “defective units” ? Ever tried Molicels ?
What I mean exactly is: [1] Have you yourself purchased and tried-out Molicel batteries ?; and [2] Do you know of (any) retailer willing to replace defective Molicel batteries known to be used for “vaping” ?
1 Like
I fail to see where this is leading. Batteries are “consumable” or nondurable products and unless it’s obvious there’s a manufacturing problem, you suck it up and replace it yourself when they wear out. AFAIK, batteries don’t fail on themselves but by user error or malfunctioning devices. And in the rare cases that there would be an issue with the battery itself, you still have nothing to say if the manufacturer’s recommending you not to use it for vaping.
2 Likes
And I respect that point of view. I’m not saying I don’t keep that in mind, but I also know that different manufacturers have different standards, ways of testing, marketing and so on. Why I trust Mooch is because he’s a vaper himself and tests different brands and models to the same standards with vaping in mind.
It’s because of people like him that you have good comparisons where you have a fair idea of what you’re dealing with when you’re purchasing your next set of batteries.
3 Likes
Right, and you (above) posted “The Mooch” himself rate the 30Q at a 15 Amperes continuous discharge current - after I pointed out that you (in likely “just a typo”) yourself stated a 20 Amperes continuous discharge current for the 30Q. Simple as that. I do not thus comprehend why the (Samsung) 15 Amp rating would be controversial ? The Samsung 30Q specifications document was published in 2014. 
2 Likes
lol
I’m not disputing the 15A rating, just saying that’s the CDR above an operating temp of 75C as tested by “The Mooch”. Below 75C, which is I’d hope every vaper’s normal operating temp, it could be considered at 20A. If you want to play it safe and still see it as 15A no matter what, that’s perfectly fine. Better safe than sorry 
2 Likes
In that best of all possible worlds, why would one care whether or not the Molicel Company “approves”?
Evidently then, it sounds like you did not experience any “incidents” with your (3500 mAH) “Fogstars” ?
How would a humble user manage to have any specific idea as to what (internal) cell temps rise to ?

1 Like
Because it’ll come to a point where vape shops will only use recommended products, or able to sell recommended products by legislation and it takes away consumer choice.
Nope, the worst that happened to a battery to me was that it wasn’t performing well anymore after more than 2 years of intensive use. I rewrap batteries, carry them in plastic containers and check that I don’t ask more from them than what they’re designed for. The hottest my batteries get is when they’re charging, which is lukewarm at best. These 10A batteries are usually charged at 500mA or less.
I’ve only used those batteries in low output 3 battery mods or a MTL device, well below a 10A output and in TC mode where an accidental push of the button might change temp but not wattage.
When it’s too hot to touch, it’s hotter than 65C. You feel that heat radiating through the mod. But short internal temperature peaks that are very damaging are obviously very hard to detect. When that happens, you should see battery performance deteriorate however and that’s a good indicator that you’re abusing your cells.
2 Likes
You recommended Molicel batteries in part on a basis that the manufacturer was “OK” (with vaping applications).
That would be entirely different than operational performance, or any governmental or private meddlings with “allowed”/“dis-allowed” ?
I suppose that you imagine “regulators” (of sorts) leaping-upon “not for vaping” statements, then ?
(In fact), there exist some FDA docs from a few years back discussing Mod power electronics. From what I recalled, (my) concern was that approved Mods would (have) to use an internal battery - which did not (in itself) appear to affect external batteries availability.
I have browser bookmarks to those FDA-related documents (presumably find-able).
It seems safe to assume that following May 12, the (FDA-regulated) industry (as we know it) will end.

1 Like
I’m not leaping to any conclusions but I’m seeing possibilities. A lot depends on how the market behaves and that’s unpredictable.
2 Likes
“Solely to Comply with UL 8139”
.
(FDA Guidance, November, 2019):
In this guidance, FDA sets out its compliance policy for premarket review requirements for two types of limited modifications to new tobacco products that were on the market as of August 8, 2016:
(1) modifications to battery-operated tobacco products solely to comply with UL 8139; and
(2) modifications to liquid nicotine products solely to comply with the Child Nicotine Poisoning Prevention Act of 2015 (CNPPA) flow restrictor requirements for liquid nicotine containers.
The cogent question is: does the FDA’s statement (above), “modifications to battery-operated tobacco products solely to comply with UL 8139”, mean that remove-able battery devices cannot (thus will not) be approve-able (in review) for use by the FDA after May 12, 2020 (less than one month from today) ?
.
(Underwriters Laboratories, October 17, 2018):
The testing requirements for UL 8139 specifically evaluate the safety of the electrical, heating, battery and charging systems. The Standard does not address devices that have removable battery cells or the consumables of the e-cigarette, including, e-liquids, vapor substances, wicks, other particulate matter.
.
What is the scope of UL 8139? UL 8139 evaluates the safety of the electrical system, including batteries, chargers, and protection circuits and controls, in electronic cigarettes and vaping devices, also known as ENDS (electronic nicotine delivery systems).
Does the standard apply to products with removable battery cells? The standard does not evaluate the performance of e-cigarettes with removable battery cells.
.
(“Baditude” posting at ECF, October 23, 2018):
UL (Underwriters Laboratories) TRIES TO ELIMINATE THE MAJOR RISK FACTORS
The new voluntary safety standard for vaping products, known as UL 8139 covers the entire electrical system: battery, charger and built-in battery management systems. A device with a removable battery cannot meet the UL 8139 standard. This eliminates the exploding battery in the pocket scenario. The device must also be designed in a way that makes it difficult for the user to change the heating coil or other major component. … It looks like this policy would eliminate mechanical mods, juice delivery attachments that will accept replaceable coils, and battery devices that use removable Li-on batteries from the e-cigarette market in the US. In other words, the only e-cigarettes that will be acceptable by UL and the FDA would be regulated battery devices with a battery management system that also uses a non-remove-able internal LiPo battery and use a “closed system” juice attachment whose wick/coils can not be removed.
.
1 Like