I may be ‘doing it wrong’ but I do that too. It’s a total PIA to mete it out when it’s cold and thick. It lives in the freezer at all times until being added to the mix.
The condensation itself really was not a worry but it definitely was a sign of stress on a product we go to great lengths to preserve!
https://www.carolinaxtract.com/cxtc-faq
Should I store Nicotine Products in the freezer?
No, Absolutely not. Damage can occur to100mg/ml nicotine products when oxides form during near-freeze/thaw cycles caused by temperature fluctuations of the freezer unit. Such fluctuations are typically caused by opening the freezer door and outside weather affecting the efficiency of the unit. Typical refrigeration temperatures, or even room temperatures are a much safer storage range than 27-33F because they are well away from freeze/thaw potential.
True! And as soon as we blend it in with the juice, it’s all downhill from there. I really don’t know if I abuse my nic, but I’ve never had any go bad on me. Could be because I use low % (3mg salts).
Thanks for posting this!
We presume that other concentrations of Nicotine in solution (would) be similarly affected. Melting points:
Nicotine: approximately -79 *C (-110 *F)
Propylene Glycol: approximately -60 *C (-76 *F)
Glycerin: approximately 18 *C (64 *F)
My (old-fashioned cooled by upper freezer-box) refrigerator likely gets a bit colder than the (water) freezing temperature - but I would bet that my 100mg/ml Nicotine suspended in 100%PG is not coming anything close to “freezing” (solidifying). In fact, when I shake the sealed containers, the contents are definitely in a liquid phase. Thus, (at least in the case of Nicotine in 100% PG), no such “freeze/thaw events” would appear at all likely to be occuring. Barring a much colder environment (ie, liquid Nitrogen cooling), I am not concerned.
Whatever small amount of permeability (may, possibly) exist in the walls of the plastic containers that the Nicotine solution is shipped in, I suspect that such activity is relatively minimal at the low storage temps. While the vendor-sealed plastic envelopes that the containers were received (and are stored) in are not perfectly invincible to gas exchange, they likely present a secondary barrier to air-exchange taking place.
The kinetics of any (possible) chemical reactions forming oxides are slowed down considerably at low temperatures [with the reactions rates roughly halving with each temperature decrease of ~10 *C (18 *F)]. Thus, mine will remain in freezer box as is - until some more legitimately convincing concerns are raised.
.
.
Nicotine having a significantly higher molecular weight (162.23 g/mol) than PG (76.09 g/mol) and VG (92.09 g/mol) - and with the high viscosity of any VG included also being a factor, (of course) one wants to raise solutions to temperatures where some vigorous physical shaking can be used to re-distribute components evenly throughout the volume of the liquid to be utilized. I keep intermediate products in my refrigerator (at ~50 * F). That (roughly) halves the rates of chemical reactions that will take place - as well as slowing (but by no means itself serving to reduce to zero) the multiplication-frequency of those nasty little microbial critters.
I just keep my working bottle in the fridge when not mixing and have no problems with color or taste. I also let it come to room temp before mixing my base.
https://www.e-cigarette-forum.com/threads/nic-base-storage-freezer-vs-room-temp-experiment.791603/
34 pages of discussion on the subject, with pictures.
Mine is staying in the freezer.
I have always practiced the tried and true method. (Freezing) I have never had a bottle anything less than perfectly clear, even after over a year and a half. I know many here keep it much longer. The only thing I have ever noticed was a slight harshness comparing my older frozen bottles to my fresh unfrozen ones.
It is nothing to me to order nicotine more often if I feel it’s better. And if it becomes hard to obtain I know how to store it if need be.
(I have not yet determined not freezing to be any better)
A (possible) “silver lining” surrounding the oxidation of Nicotine (and subsequent consumption thereof).
Folks may already be familiar with this - while the hypothesis may well not pertain to the aesthetic elements of vaping, the pharmacological effects related to Nicotine absorbed appear to be potentially significant (and beneficial) where it comes to the pharmacokinetics of absorbed Nicotine (with some possible down-sides where efficiency of hepatic detoxification of certain specific toxins is concerned). An interesting subject.
Electronic Cigarettes: The Nicotyrine Hypothesis (2015)
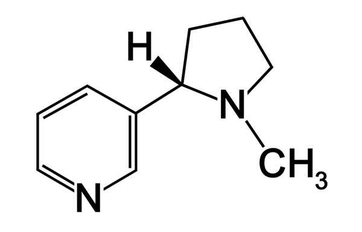
Nicotine:
Nicotyrine:
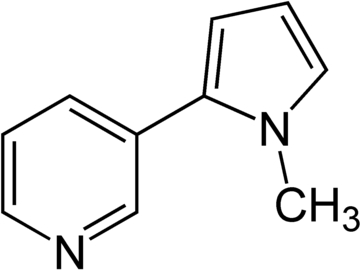
.

Image source here.
Very interesting read, thanks.
Indeed. One wonders to what extent the oxidation (in part) of Nicotine to Nicotyrine may (possibly) enter into the myriad of impressions surrounding the “aging” of e-juices (or just Nicotine in PG or VG solution alone). The problem with analysis of the Nicotyrine relation seems to be that there are so many different possible interactions and reactions between various components of e-juice over time and temperature that aesthetic elements of “taste” seem by nature “cloudy”. As well, the pharmacology of Nicotine in an individual is a hard thing to get a meaningful anecdotal reporting handle on. In both cases, the variations of perceived “taste” as well as effects of Nicotine can vary significantly throughout the course of one’s waking/vaping day. Aesthetic experiences are ever varying things that (like consciousness) we can only experience in the singular, and never the plural, sense. Thus, much like visual or auditory aesthetics, these experiences remain implicitly subjective (as well as variable, even in the individual case). Generalizations are potentially misguided. The best that I’ve been able to do is to slowly gain a “bio-averaged” feel for this or that set of parameter values.
I re-read the above discussion. Hmmm
Whew. I am impressed and slight confused with the different, very intelligent, words. Guess I’ll assume the meaning based on context.
I like to keep mine in the freezer. I do not split it up when I buy big bottles (haven’t had troubles yet). I also take the bottle out to sit overnight(I use VG based nic)
I have 2 15ml glass bottles of nic sitting out at all times. It will always amaze me how many roads there are that lead to the same destination! Good read!
We presume that other concentrations of Nicotine in solution (would) be similarly affected. Melting points:
Nicotine: approximately -79 *C (-110 *F)
Propylene Glycol: approximately -60 *C (-76 *F)
Glycerin: approximately 18 *C (64 *F)
My (old-fashioned cooled by upper freezer-box) refrigerator likely gets a bit colder than the (water) freezing temperature - but I would bet that my 100mg/ml Nicotine suspended in 100%PG is not coming anything close to “freezing” (solidifying). In fact, when I shake the sealed containers, the contents are definitely in a liquid phase. Thus, (at least in the case of Nicotine in 100% PG), no such “freeze/thaw events” would appear at all likely to be occuring. Barring a much colder environment (ie, liquid Nitrogen cooling), I am not concerned.
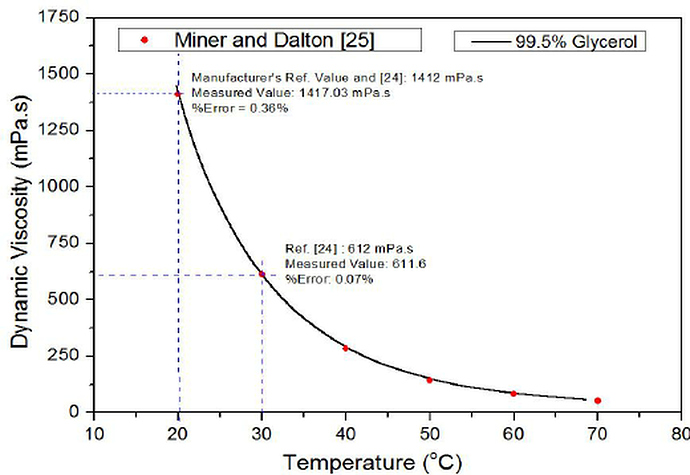
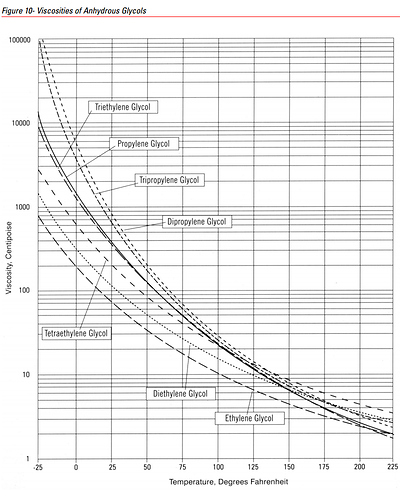
Here is one particular scenario to likely be avoided (that is possible with close to pure Glycerol, anyway):
Below are a couple of graphics showing Viscosity (of Glycerol, as well as various Gylcols, including PG):
Source: Search | Dow Inc.
Having a rough look at ~86 *F (~30 *C), Glycerol has ~17 times the Viscosity that Propylene Glycol has.
I find mentions of shelf-life (under 40 *C) of ~2 Years for VG, and for PG (which should be less reactive).
I use VG based nic and it never actually freezes in my freezer.
I have used nic from my freezer that was over 3 years old and it was perfect, as if it was brand new.
No science involved in my statement, just my own experience.
Hiliq’s recommended storage temp is 3C to 8C.
With liquid Nitrogen systems, Glycerol lattices sporting levorotatory Nicotine molecules having methyl groups uniformly aligned to magnetic North can reliably provide consistently better than perfect results:

![]()
I’m not even going to try to understand the science bit, and definitely don’t understand “better than perfect”, surely that’s a contradiction in terms, but what do I know, I’m just a reasonably normal human. ![]()